Iimveliso
Ikhabhon Tetrafluoride
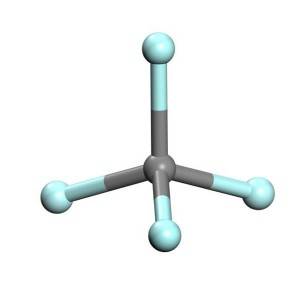
I-Tetrafluoromethane, nekwaziwa ngokuba yi-carbon tetrafluoride, yeyona fluorocarbon elula (CF4).Inamandla okudibanisa kakhulu ngenxa yendalo yebhondi ye-carbon-fluorine.Inokuthi ihlelwe njenge-haloalkane okanye i-halomethane.Ngenxa yeebhondi ezininzi ze-carbon-fluorine, kunye ne-electronegativity ephezulu ye-fluorine, ikhabhoni ekwi-tetrafluoromethane inexabiso elibalulekileyo elilungileyo eliqinisayo kwaye lifinyeze iibhondi ezine ze-carbon-fluorine ngokubonelela ngophawu olongezelelweyo lwe-ionic.I-Tetrafluoromethane yigesi enamandla ye-greenhouse.
I-Tetrafluoromethane ngamanye amaxesha isetyenziswa njengefriji yobushushu obuphantsi.Isetyenziswa kwi-electronics microfabrication yodwa okanye idityaniswe ne-oksijini njenge-plasma etchant ye-silicon, i-silicon dioxide, kunye ne-silicon nitride.
| Ifomula yemichiza | CF4 | Ubunzima bemolekyuli | 88 |
| Inombolo yeCAS. | 75-73-0 | I-EINECS No. | 200-896-5 |
| Indawo yokunyibilika | -184℃ | Indawo yeBoling | -128.1℃ |
| ukunyibilika | Ayinyibiliki emanzini | Ukuxinana | 1.96g/cm³(-184℃) |
| Imbonakalo | Irhasi engenambala, engenavumba, engatshayo, ecinezelekayo | Isicelo | isetyenziswe kwinkqubo yokufakwa kweplasma kwiisekethe ezahlukeneyo ezidibeneyo, kwaye isetyenziswe njengerhasi yelaser, ifriji njl. |
| Inombolo ye-ID ye-DOT | UN1982 | I-DOT/IGAMA LOKUTHUNYELWA KWE-IMO: | I-Tetrafluoromethane, iGesi eQiniweyo okanye eFrijiweyo R14 |
| Iklasi ye-DOT yengozi | Iklasi 2.2 |
| Into | Ixabiso, ibakala I | Ixabiso, ibakala II | Iyunithi |
| Ubunyulu | ≥99.999 | ≥99.9997 | % |
| O2 | ≤1.0 | ≤0.5 | ppmv |
| N2 | ≤4.0 | ≤1.0 | ppmv |
| CO | ≤0.1 | ≤0.1 | ppmv |
| CO2 | ≤1.0 | ≤0.5 | ppmv |
| SF6 | ≤0.8 | ≤0.2 | ppmv |
| Ezinye ii-fluorocarbons | ≤1.0 | ≤0.5 | ppmv |
| H2O | ≤1.0 | ≤0.5 | ppmv |
| H2 | ≤1.0 | —- | ppmv |
| Ubumuncu | ≤0.1 | ≤0.1 | ppmv |
| *ezinye iifluorocarbons zibhekisa kuC2F6, C3F8 | |||
Amanqaku
I-1) yonke idatha yobugcisa eboniswe ngasentla yereferensi yakho.
2) enye ingcaciso yamkelekile kwingxoxo eqhubekayo.