Alaabta
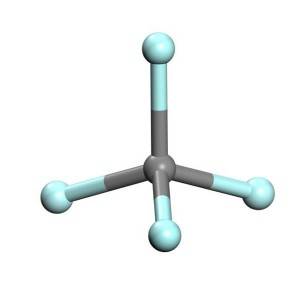
Kaarboon Tetrafluoride
Tetrafluoromethane, oo sidoo kale loo yaqaan carbon tetrafluoride, waa fluorocarbon ugu fudud (CF4).Waxay leedahay awood isku xidhid aad u saraysa taas oo ay ugu wacan tahay dabeecadda curaarta kaarboon-fluorine.Waxa kale oo loo kala saari karaa sida haloalkane ama halomethane.Sababtoo ah curaarta kala duwan ee kaarboon-fluorine, iyo electronegativity ugu sarreeya ee fluorine, kaarboonka ku jira tetrafluoromethane wuxuu leeyahay qayb weyn oo togan oo xoojisa oo soo gaabinaysa afarta kaarboon-fluorine bonds iyadoo la siinayo dabeecad ionic dheeraad ah.Tetrafluoromethane waa gaas aqalka dhirta lagu koriyo oo awood leh.
Tetrafluoromethane waxaa mararka qaarkood loo isticmaalaa qaboojiye heerkul hoose ah.Waxaa loo isticmaalaa qalabka elektarooniga ah oo keliya ama lagu daro ogsijiinta sida balaasmaha silikoon, silicon dioxide, iyo silicon nitride.
| Kiimikada caanaha | CF4 | Miisaanka molecular | 88 |
| CAS Maya. | 75-73-0 | EINECS Maya. | 200-896-5 |
| barta dhalaalaysa | -184 ℃ | Barta booga leh | -128.1 ℃ |
| milmayn | Aan biyo ku milmin | Cufnaanta | 1.96g/cm³ (-184℃) |
| Muuqashada | Midab-la'aan, ur aan lahayn, aan guban, gaas la isku cadaadi karo | Codsiga | loo isticmaalo habka etching plasma ee wareegyada isku dhafan ee kala duwan, oo loo isticmaalo sidoo kale gaaska laysarka, qaboojiyaha iwm. |
| Lambarka aqoonsiga DOT | UN1982 | Magaca DOT/IMO: | Tetrafluoromethane, Gaaska la tuujiyey ama qaboojiyaha R14 |
| Heerka Khatarta DOT | Fasalka 2.2 |
| Shayga | Qiimaha, fasalka I | Qiimaha, fasalka II | Unug |
| daahirnimo | ≥99.999 | ≥99.9997 | % |
| O2 | ≤1.0 | ≤0.5 | ppmv |
| N2 | ≤4.0 | ≤1.0 | ppmv |
| CO | ≤0.1 | ≤0.1 | ppmv |
| CO2 | ≤1.0 | ≤0.5 | ppmv |
| SF6 | ≤0.8 | ≤0.2 | ppmv |
| fluorocarbons kale | ≤1.0 | ≤0.5 | ppmv |
| H2O | ≤1.0 | ≤0.5 | ppmv |
| H2 | ≤1.0 | —— | ppmv |
| Aysiidhka | ≤0.1 | ≤0.1 | ppmv |
| * fluorocarbons kale waxay tixraacaan C2F6C3F8 | |||
Xusuusin
1) Dhammaan xogta farsamada ee kor lagu tilmaamay waa tixraacaaga.
2) Faahfaahin dheeraad ah ayaa lagu soo dhaweynayaa dood dheeraad ah.