
Products
Manufacturer for Potassium Perchlorate For Fireworks - Ammonium perchlorate – YANXA
Manufacturer for Potassium Perchlorate For Fireworks - Ammonium perchlorate – YANXA Detail:
| Molecular formula: |
NH4ClO4 |
Molecular weight: |
117.50 |
| CAS No. |
7790-98-9 |
RTECS No. |
SC7520000 |
| UN No.: |
1442 |
|
Ammonium perchlorate is an inorganic compound with the formula NH₄ClO₄. It is a colorless or white solid that is soluble in water. It is a powerful oxidizer. Combined with a fuel, it can be used as a rocket propellant.
Uses: mainly used in rocket fuel and smokeless explosives, besides, it is widely used in explosives, photographic agent, and analytical reagent.
1) anti-caked by SDS

2) anti-caked by TCP

Prior to working with ammonium perchlorate, you should be trained on its proper handling and storage.
Ammonium perchlorate is a strong oxidizer; and mixtures with sulfur, organic materials, and finely divided metals are explosive and friction and shock sensitive.
Ammonium perchlorate must be stored to avoid contact with oxidizing agents (such as perchlorates peroxides. Permanganates, chlorates nitrates, chlorine, bromine and fluorine since violent reactions occur.
Ammonium perchlorate is not compatible with strong reducing agents: strong acids (such as hydrochloric. Sulfuric and nitric) metals (such as aluminum. Copper, and potassium); metal oxides: phosphorous: and combustibles.
Wherever ammonium perchlorate is used, handled manufactured, or stored, use explosion-proof electrical equipment and fittings.
Precautions
Keep away from heat. Keep away from sources of ignition. Keep away from combustible material. Empty containers pose a fire risk, evaporate the residue under a fume hood. Ground all equipment containing material.
Do not breathe dust. Take precautionary measures against electrostatic discharges. Wear suitable protective clothing. In case of insufficient ventilation, wear suitable respiratory equipment. If you feel unwell, seek medical attention and show the label when possible. Avoid contact with skin and eyes. Keep away from incompatibles such as reducing agents, combustible materials, organic materials, acids.
Storage
Keep container tightly closed. Keep container in a cool, well-ventilated area. Separate from acids, alkalies, reducing agents and combustibles.
Product detail pictures:
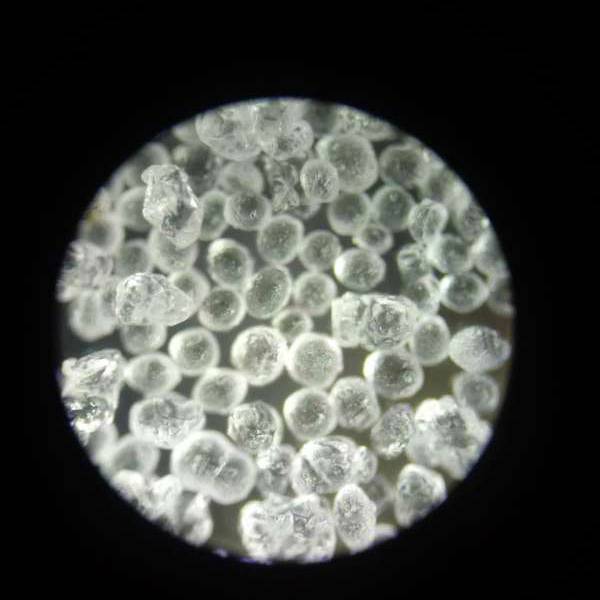
Related Product Guide:
We're proud from the higher client fulfillment and wide acceptance due to our persistent pursuit of high quality both on product and service for Manufacturer for Potassium Perchlorate For Fireworks - Ammonium perchlorate – YANXA , The product will supply to all over the world, such as: Senegal, Croatia, Qatar, We'd like to invite customers from abroad to discuss business with us. We can provide our clients with high quality products and excellent service. We are sure that we will have good cooperative relationships and make a brilliant future for both parties.
Goods just received, we are very satisfied, a very good supplier, hope to make persistent efforts to do better.




