Kayayyaki
Carbon Tetrafluoride
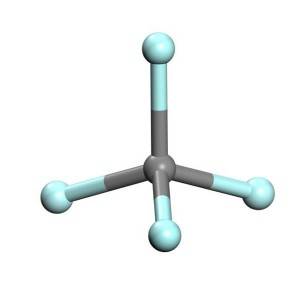
Tetrafluoromethane, kuma aka sani da carbon tetrafluoride, shine mafi sauƙin fluorocarbon (CF4).Yana da ƙarfin haɗin gwiwa sosai saboda yanayin haɗin carbon-fluorine.Hakanan ana iya rarraba shi azaman haloalkane ko halomethane.Saboda nau'ikan haɗin carbon-fluorine da yawa, da mafi girman electronegativity na fluorine, carbon a cikin tetrafluoromethane yana da ingantaccen caji mai ƙarfi wanda ke ƙarfafawa da rage haɗin haɗin carbon-fluorine guda huɗu ta hanyar samar da ƙarin halayen ionic.Tetrafluoromethane shine iskar gas mai ƙarfi.
Tetrafluoromethane wani lokaci ana amfani dashi azaman sanyi mai ƙarancin zafin jiki.Ana amfani dashi a cikin microfabrication na lantarki kadai ko a hade tare da oxygen a matsayin wani abu na plasma don silicon, silicon dioxide, da silicon nitride.
| Tsarin sinadaran | CF4 | Nauyin kwayoyin halitta | 88 |
| CAS No. | 75-73-0 | EINECS No. | 200-896-5 |
| Wurin narkewa | -184 ℃ | Ma'ana mai ƙarfi | -128.1 |
| narkewa | Mara narkewa a cikin ruwa | Yawan yawa | 1.96g/cm³ (-184℃) |
| Bayyanar | Gas mara launi, mara wari, mara ƙonewa, iskar gas | Aikace-aikace | Ana amfani da shi a cikin tsarin etching na plasma don haɗaɗɗun da'irori daban-daban, kuma ana amfani dashi azaman iskar gas, refrigerant da sauransu. |
| Lambar ID DOT | UN1982 | DOT/IMO SUNAN SHIRI: | Tetrafluoromethane, Matsi ko Refrigerant Gas R14 |
| Babban darajar DOT Hazard | Darasi na 2.2 |
| Abu | daraja, daraja I | daraja, daraja II | Naúrar |
| Tsafta | ≥99.999 | ≥99.9997 | % |
| O2 | ≤1.0 | ≤0.5 | ppmv |
| N2 | ≤4.0 | ≤1.0 | ppmv |
| CO | ≤0.1 | ≤0.1 | ppmv |
| CO2 | ≤1.0 | ≤0.5 | ppmv |
| SF6 | ≤0.8 | ≤0.2 | ppmv |
| Sauran fluorocarbons | ≤1.0 | ≤0.5 | ppmv |
| H2O | ≤1.0 | ≤0.5 | ppmv |
| H2 | ≤1.0 | -- | ppmv |
| Acidity | ≤0.1 | ≤0.1 | ppmv |
| * sauran fluorocarbons suna nufin C2F6,C3F8 | |||
Bayanan kula
1) duk bayanan fasaha da aka nuna a sama don tunani ne.
2) madadin bayani yana maraba don ƙarin tattaunawa.